3 Subatomic Particles That Make Up an Atom
Protons and neutrons are nucleons. A subatomic particle is a particle a size smaller than that of an atom.

The Chemistry Of Life Objectives What Three Subatomic Particles Make Up Atoms How Are All The Isotopes Of An Element Similar What Are The Two Types Ppt Download
Protons neutrons and electrons.
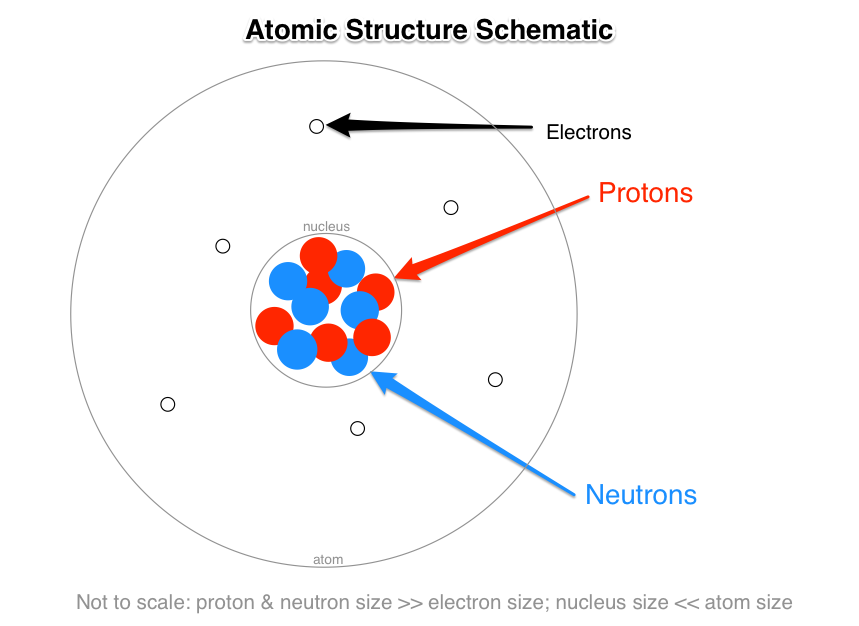
. An easy way to remember this is to remember that both proton and positive start with the letter P Neutrons have no electrical charge. Neutrons have no electrical charge. Nucleus of an Atom The nucleus of the atom was discovered in the year 1911 by Ernest Rutherford a physicist from New Zealand.
The three common subatomic particles are the proton the neutron and the electron. FAQs qnadmin December 23 2021. Which is a positively charged particle in an atom.
Protons neutrons and electrons are the three main subatomic particles found in an atom. An easy way to remember this is to remember that both proton and positive start with the letter P Neutrons have no electrical charge. What are 3 subatomic particles that make up an atom.
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each. The subatomic particles of protons and neutrons are found in the nucleus of an atom. As a result the existence of various types of matter around us is due o the presence of atoms in them.
The value of difference in electronegativity between two atoms in a covalent bond is less than 17. Protons have a positive charge. The number of protons also determines the identity of the atom its atomic number.
Electrons have negative - charge. Protons neutrons and electrons. The components of the atoms are known as subatomic particles and usually include the proton the electron and the neutron.
Two of the subatomic particles have electrical charges. Protons have a positive charge. 3 rows Protons neutrons and electrons make up the subatomic particles of an atom.
Given that these particles make up atoms they are often referred to as subatomic particles. Atomic structure and the periodic table. Neutrons have no electrical charge and are also in the nucleus.
What subatomic particles determine the atomic number. Protons neutrons and electrons are the three main subatomic particles found in an atom. The protons charge is 1 and is balanced out in a neutral atom by the electrons -1 charge.
Protons neutrons and electrons. Protons neutrons and electrons. The center of the atom is called the nucleus.
The three main subatomic particles that form an atom are protons neutrons and electrons. Atoms of each element contain a characteristic number of protons. Given that these particles make up atoms they are often referred to as subatomic particles.
The center of the atom is called the nucleus. There are three subatomic particles. Electrons are the least massive of an atoms constituent particles with a mass of 911 x 1031 kg and a size too small to be measured by current.
While its name originally referred to a particle that couldnt be divided any morethe smallest thing possiblewe now know that each atom is generally made up of smaller particles. This 22 words question was. Subsequently one may also ask what are the three subatomic particles of an atom and where are they located.
The three main subatomic particles that form an atom are protons neutrons and electrons. Protons are positively charged and are located in the nucleus. The neutron having no charge is neutral.
Protons neutrons and electrons To see more answers head over to College Study Guides. Protons neutrons and electrons are the three main subatomic particles found in an atom. First lets learn a bit about protons and neutrons and then we will talk about electrons a little later.
3 main subatomic particles that form the atom Protons neutrons and electrons At the center of the atom is the Nucleus Covalent bonds form when two atoms have a very small nearly insignificant difference in electronegativity. Protons have a positive charge while electrons have a negative charge. Protons have a positive charge.
Protons neutrons and electrons are the three main subatomic particles found in an atom. Protons neutrons and electrons. Protons have a positive charge.
They also help contribute to the atomic weight. Protons and neutrons make up the nucleus of an atom. There are three subatomic particles.
Click to see full answer. The three subatomic particles that make up the atom are protons and neutrons inside the nucleus of the atom and electrons which are located in the electron cloud surrounding the atomic nucleus. Protons have a positive charge while electrons have a negative charge.
The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. There are three subatomic particles. The majority of an atoms mass comes from the protons and neutrons that make up its nucleus.
Which subatomic particles make up most of the mass of the atom. Protons electrons and neutrons are the three subatomic particles that typically make up an atom. Two of the subatomic particles have electrical charges.
An easy way to remember this is to remember that both proton and positive start with the letter P Neutrons have no. 3 Objective 1 Subatomic Particles Electron e -1 000055 0 Neutron n 0 100867 1 Proton p 1 100728 1 Mass Number Mass daltons Name Symbol Charge 4 Objective 2 Describe the basic structure of the atom and be able to define the. An atom itself is made up of three tiny kinds of particles called subatomic particles.
Who discovered subatomic particles with no charge. Atoms are the fundamental components of matter.
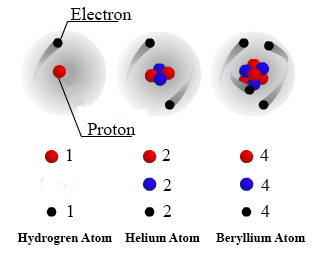
Nondestructive Evaluation Physics Atomic Elements
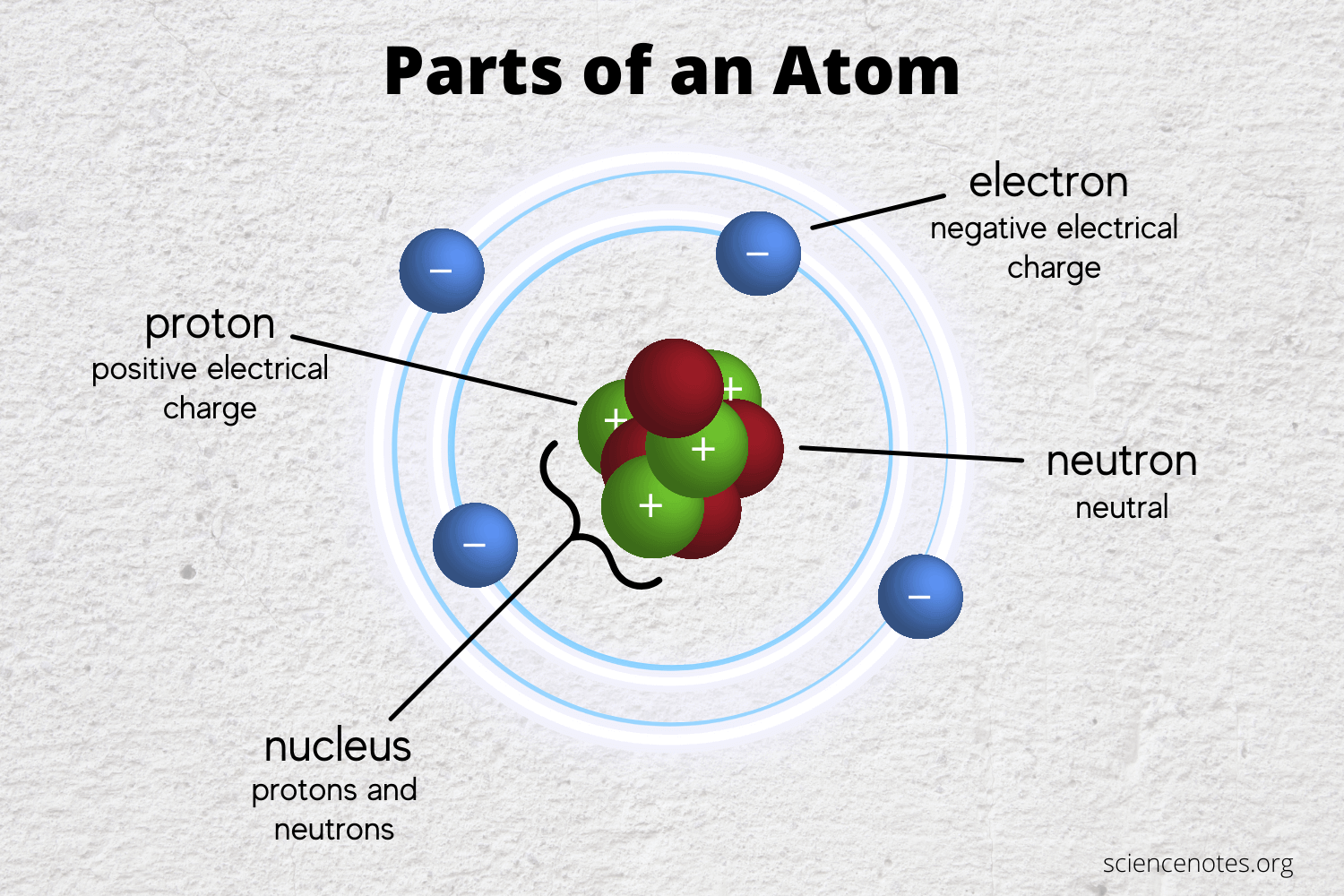
The Famous Types Of Subatomic Particles Praxilabs

What Are The Names Charges And Locations Of The Three Types Of Subatomic Particles That Make Up An Atom Socratic
No comments for "3 Subatomic Particles That Make Up an Atom"
Post a Comment